CUPERTINO, Calif. & GOYANG-SI, South Korea
Zepp Health Corp. (NYSE: ZEPP), and Rouumtech Co., Ltd., today announced the expansion of an agreement initially reached with predecessor Aspen Imaging Healthcare, to private label a customized Europa™ portable X-ray system, along with the company’s entire line of X-ray imaging products, for sales in China.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210304005323/en/
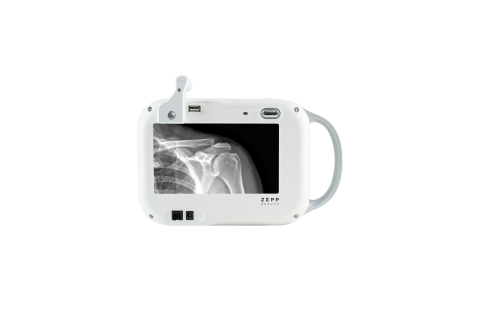
Customized Europa™ portable X-ray system by Zepp Health and Rouumtech (Photo: Business Wire)
This step builds on the relationship initially announced in August 2020. Prior to marketing the Europa and other Rouumtech X-ray products, Zepp Health will help Rouumtech obtain China market clearance by the National Medical Products Administration (NMPA). The companies will continue to move towards working together in engineering, such as leveraging Zepp Health’s artificial intelligence (AI) expertise for product development, and additional global market opportunities.
“Zepp Health’s broad mission is to connect health with technology,” said Mike Yeung, Zepp Health’s chief operating officer, based in Cupertino, California. “In 2020, we launched relationships to build a new industrial healthcare revenue base to supplement our global position in consumer health technology. We are beginning to leverage new medical imaging technologies such as those by Rouumtech, that are disrupting the locations, applications and costs of medical imaging.”
“We are very excited to work together with Zepp Health to bring our compact, portable X-ray systems to the Chinese market,” said Jung Ho Park, CEO of Rouumtech. “We’re excited to see the direction that Zepp Health is taking to enter the medical imaging industry, and we are very honored to be a partner.”
“Partnerships, such as Rouumtech allow Zepp Health to expand its ability to deliver health technology services globally,” said Tim Houchin, Zepp Health’s vice president of business and corporate development. “The medical imaging innovation from Rouumtech, combined with our miniaturization engineering and user interface expertise can advance how clinicians provide medical care to patients.”
About Zepp Health Corporation (NYSE: ZEPP)
Zepp Health changed its name from Huami Corp. (HMI) on February 25, 2021 to emphasize its health focus with a name that more easily crosses languages and cultures globally. The company’s mission continues to be connecting health with technology. Since its inception in 2013, Zepp Health has developed a platform of proprietary technology including AI chips, biometric sensors, and data algorithms, which drive a broadening line of smart health devices for consumers, data analytics services for population health, and industrial medical technology for diagnostics and care delivery. Zepp Health is one of the largest global developers of smart wearable health and consumer fitness devices, shipping 41 million units in 2019. Zepp Health Corp. is based in Hefei, China, with U.S. operations, Zepp Health USA, based in Cupertino, Calif.
About Rouumtech Co, Ltd.
Rouumtech is a medical imaging innovator, pioneering new formats and applications of X-Ray imaging technology. The company is headquartered in Goyang-si, Gyeonggi-do, KOREA. For more information, please visit www.rouumtech.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210304005323/en/
CONTACT
For Zepp Health media inquiries:
Lydia Huang, lydia.huang@huami-usa.com, c: 407-800-5625
For Zepp Health investors:
U.S. – Brad Samson, brad.samson@huami-usa.com, c: 714-955-3951
China – Grace Zhang, zhangyujia@huami.com
For Rouumtech media inquiries:
Yosep Park, yspark@livermoretechusa.com





