TOKYO & NEW YORK & DÜSSELDORF, Germany

AW Technologies and Asahi Kasei Medical, a core operating company of the Asahi Kasei Group, have entered into an exclusive distribution agreement in Japan for AW Technologies’ TrachFlush™ device. AW Technologies is currently in the process of medical device registration of the TrachFlush in Japan, with market launch targeted in fiscal 2024.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240403877269/en/
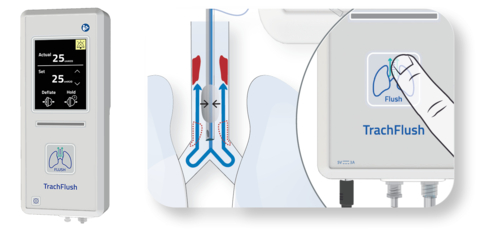
The TrachFlush and a unique cuff pressure control aligned with ventilator flow. (Graphic: Business Wire)
Developed by AW Technologies based on an invention by an intensivist, the TrachFlush is a medical device to reduce the discomfort of ventilated patients while lightening the workload on healthcare professionals during tracheal suctioning. The TrachFlush supports airway secretion (phlegm) management with a unique cuff pressure control system, and has received the CE Mark in Europe in 2020 and FDA clearance in the United States in 2023. In Japan, marketing and sale is to be performed by Asahi Kasei Medical.
With the push of a button, the TrachFlush deflates the cuff and utilizes the ventilator air pressure during an inspiratory cycle, and re-inflates the cuff before the cycle completes.
Ken Shinomiya, President of Asahi Kasei Medical, stated, “We are delighted to add the TrachFlush to our product lineup in the intensive care field, where we have strength in the area of blood purification. By leveraging our experience and know-how in blood purification, we believe that this will deliver diverse value, such as enhancing patients’ QOL and reducing the burden on healthcare workers.”
Adam Hansen, CEO of AW Technologies, commented, “We are very pleased and excited to announce our partnership with Asahi Kasei Medical. This partnership is very important for AW Technologies, and Asahi Kasei Medical will play a very important role in our expansion of TrachFlush globally. We believe the TrachFlush will have a great impact in the ICU – not only by preventing ventilator-associated pneumonia, but also by non-invasively preventing the accumulation of secretion in the airways.”
About AW Technologies ApS
AW Technologies is a Danish medical device company, established in 2018, focused on developing disruptive and innovative airway management solutions for mechanically ventilated patients in the ICU, including TrachFlush. For further information, please visit www.trachflush.com and www.awtechnologies.dk.
About Asahi Kasei and Asahi Kasei Medical
The Asahi Kasei Group contributes to life and living for people around the world. Since its foundation in 1922 with ammonia and cellulose fiber business, Asahi Kasei has consistently grown through the proactive transformation of its business portfolio to meet the evolving needs of every age. With more than 48,000 employees worldwide, the company contributes to sustainable society by providing solutions to the world’s challenges through its three business sectors of Material, Homes, and Health Care. Asahi Kasei Medical is a core operating company in the Health Care sector. Its blood purification business comprises devices and systems for dialysis and therapeutic apheresis, and its bioprocess business comprises equipment for the manufacture of biotherapeutics, biosafety testing services, and biologics CDMO operations. For more information, visit www.asahi-kasei.com and www.asahi-kasei.co.jp/medical/en/.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240403877269/en/
CONTACT
North America Contact:
Asahi Kasei America Inc.
Christian OKeefe
christian.okeefe@ak-america.com
Europe Contact:
Asahi Kasei Europe GmbH
Sebastian Schmidt
sebastian.schmidt@asahi-kasei.eu





