OSAKA, Japan

Gunze Limited (Headquarters: Osaka, Japan, President: Toshiyasu Saguchi) [TOKYO: 3002] is pleased to announce that Gunze has obtained medical device approval to manufacture and sell TENALEAF™, the first sheet-type absorbable adhesion barrier made in Japan. The company is planning to launch the product in March 2022 through its subsidiary “Gunze Medical Japan” in the Japanese market.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220223005048/en/
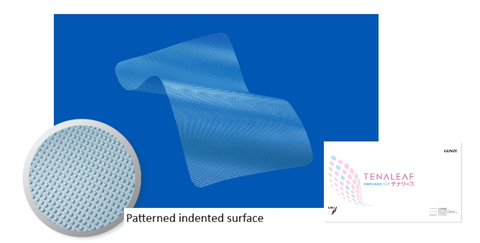
Figure: TENALEAF absorbable adhesion barrier (Graphic: Business Wire)
As the average life expectancy increases, the possibility of undergoing multiple surgeries in one’s lifetime also rises. The use of an absorbable adhesion barrier is expanding to reduce postoperative organ adhesions (Note) which can lead to difficult second and subsequent surgeries. Also, in order to improve the patient’s clinical outcome, laparoscopic surgery has recently been growing.
Gunze developed its absorbable adhesion barrier by applying proprietary absorbable material technology with the goal of providing an innovative solution in this area. This device provides surgeons with a new option during open surgery and minimally invasive surgery, which can enable easy manipulation, provide moderate adhesive strength for repositioning and conformable placement. The company strives to expand its recognition by informing the new product features and benefits to surgeons and their patients.
(Note)What are “postoperative organ adhesions”?
They typically occur after surgical intervention, where organs and/or tissue adhere to each other during the tissue remodeling process. It occurs frequently regardless of the surgical procedure, and may cause postoperative complications such as intestinal obstruction and infertility, which may require removal of adhesions in the second or subsequent surgery.
About Gunze
Gunze was founded in Kyoto, Japan in 1896, and today operates diverse businesses as a leading developer and manufacturer of Medical Devices, Plastic Films, Engineering Plastics, Electronic Components, Apparel, and various other segments. Gunze employs more than 5,800 people worldwide throughout 10 countries.
The Medical Device business, established in 1985, has a substantial footprint in over 35 countries. Its head office and manufacturing facility are located in Kyoto, Japan, and has subsidiaries in the U.S., EU, and China. By applying innovative fiber and polymer processing technologies, Gunze manufactures a comprehensive range of medical products focused on bioabsorbable and biocompatible materials such as skin substitutes, tissue reinforcement felt, bone fixation devices, dural substitutes, and suture thread.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220223005048/en/
CONTACT
Gunze Limited, Corporate Communication Department
Shinji Nonaka
TEL: +81-6-6348-1314
HP: https://www.gunze.co.jp/english/





