MADISON, Wis.

Researchers from Johns Hopkins University have shown that a new panel of long mononucleotide repeats (LMR) might offer advantages over traditional microsatellite instability (MSI) detection methods in certain types of solid tumors. The study, published in the February issue of Journal of Molecular Diagnostics, is the first to use the PCR-based Promega LMR MSI Analysis System to detect MSI in endometrial, prostate and other cancers.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220202005671/en/
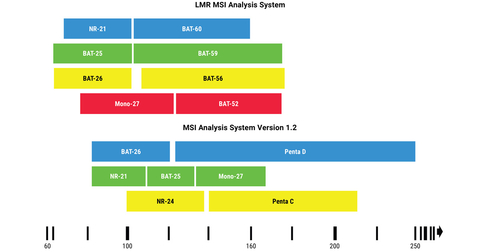
A study published in Journal of Molecular Diagnostics states that the researchers will continue using the current gold standard for PCR-based microsatellite instability (MSI) testing, the Promega MSI Analysis System, Version 1.2, for detecting MSI in colorectal cancer, but they plan to adopt the Promega LMR MSI Analysis System for endometrial, prostate and other non-GI cancers. (Graphic: Business Wire)
Detecting Microsatellite Instability
MSI analysis measures changes in the length of specific DNA sequences that occur because of a deficiency in a cell’s DNA mismatch repair system. MSI status is a valuable indicator for predicting whether an individual might respond to immune checkpoint inhibitor therapy. The Promega MSI Analysis System, Version 1.2 is the current gold standard for PCR-based MSI testing and has been relied upon by global clinical researchers for nearly two decades. The assay amplifies a panel of five mononucleotide repeat markers, each composed of 21-27 repeated adenine bases. This technology is used in the OncoMate™ MSI Dx Analysis System, which is available as a CE-IVD in Europe and an FDA-cleared IVD medical device in the United States.
The LMR MSI Analysis System, launched in February 2021, consists of eight markers – four of the gold standard markers, plus four novel LMR markers that have 52-60 repeated adenine bases each. Longer markers are typically more “unstable,” or prone to replication errors, so it has been hypothesized that this new panel of markers might offer greater sensitivity for MSI detection in cancers where the results are otherwise inconclusive or ambiguous.
LMR Analysis Research
The research group led by Dr. James Eshleman, M.D., Ph.D. validated the LMR MSI panel using colorectal, endometrial, and prostate tumor samples, as well as a variety of other tumor samples with inconclusive results in previous MSI testing.
Statistics for the LMR MSI panel include:
- Colorectal cancer: 100% accuracy, 100% clinical sensitivity, 100% clinical specificity
- Endometrial cancer: 98% accuracy, 98% clinical sensitivity, 100% clinical specificity
- Prostate cancer: 75% clinical sensitivity
- Other cancers: 100% concordance between LMR MSI and MSI V1.2 in 22 samples previously designated MSI-High
“The new LMR panel clearly offers unique benefits in certain cancer types,” says Jeff Bacher, Senior Research Scientist at Promega and co-author on the study. “These results are encouraging for the detection of microsatellite instability in samples that were previously difficult or impossible.”
The paper states that the Johns Hopkins researchers will continue using the MSI Analysis System, Version 1.2 for detecting MSI in colorectal cancer, and they have adopted the LMR MSI Analysis System for endometrial, prostate and other non-GI cancers.
To learn more about the LMR MSI Analysis System, visit www.promega.com/LMRAnalysis
Promega Clinical Research Program
This study was supported in part by the Promega Clinical Research Program (PCRP), a global initiative that supports researchers using Promega technologies to advance clinical diagnostics. This is the first publication to result from a PCRP partnership, and seven more projects are currently benefitting from Promega products and expertise.
About Promega Corporation
Promega Corporation is a leader in providing innovative solutions and technical support to the life sciences industry. The company’s portfolio of over 4,000 products support a range of life science work across areas such as cell biology; DNA, RNA and protein analysis; drug development; human identification and molecular diagnostics. For over 40 years these tools and technologies have grown in their application and are used today by scientists and technicians in labs for academic and government research, forensics, pharmaceuticals, clinical diagnostics and agricultural and environmental testing. Promega is headquartered in Madison, WI, USA with branches in 16 countries and over 50 global distributors. For more information, visit www.promega.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20220202005671/en/
CONTACT
Penny Patterson
VP, Corporate Affairs
Promega Corporation
Phone: (608) 274-4330
E-mail: penny.patterson@promega.com





