DANVERS, Mass.
NeuroLogica’s BodyTom Elite, the company’s mobile, full-body, 32-slice CT (computed tomography) scanner, is now available for purchase in Japan.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211116005233/en/
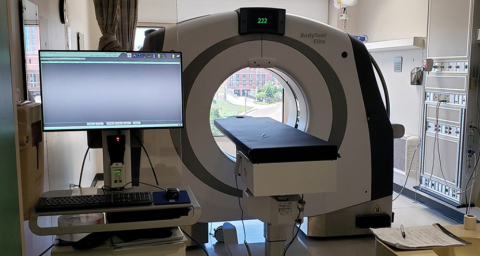
NeuroLogica’s BodyTom Elite, the company’s full-body, 32-slice CT (computed tomography) scanner, is now available for purchase in Japan.
“The expansion of the market for the BodyTom Elite, which transforms any hospital room into an advanced imaging suite, means more patients will have access to leading-edge diagnostic imaging,” said Jason Koshnitsky, Senior Director, Global Sales and Marketing for NeuroLogica. “The BodyTom Elite seamlessly integrates into any OR, Neurosurgery, Radiation Oncology or Radiology Suite, and provides a more accurate, sharper image.”
BodyTom Elite CT systems are clinically used in these environments in more than 20 countries.
Japan has one of the highest CT scanners per unit population in the world. BodyTom Elite offers additional flexibility allowing point-of-care CT imaging, and reduces risks associated with moving critically ill patients to radiology.
In the world of advanced imaging, millimeters can be the difference when it comes to delivering an accurate diagnosis or treatment. BodyTom Elite delivers that capability, with features including:
- New detector material Gadolinium Oxysulfide Detector that has better scintillation properties than the previous detector material
- Battery operation for use in any environment
- Charging from standard wall outlet
- Self-shielded with ability to use in most rooms
- Improved distribution of power in the system with new design of the lithium batteries
The BodyTom Elite software offers a slew of features, including:
- Comprehensive cybersecurity features with built-in tools for protecting against cyber threats from external attacks
- Low dose lung cancer screening
- Contrast capability, which allows use of CT angiography and perfusion imaging utilizing bolus tracking and manual start
- Metal Artifact Reduction (MAR) improving metal implants and trauma cases
- A noise-reduction algorithm, which will vastly improve image quality
- Automatic exposure control, which minimizes the amount of radiation by factoring the thickness and density of a patient
- Wireless access point providing better security and stability of wireless communication by creating a standalone wireless network for the scanner compared to the existing Ad-Hoc environment
BodyTom Elite is XR-29 (MITA Smart Dose) compliant and as such includes four key features of CT equipment that enable optimization or management of radiation dose delivery – dose structured reporting, CT dose check, AEC and pediatric and adult reference protocols.
The BodyTom Elite is self-shielded, can accommodate patients of most sizes, and its combination of rapid scan time, flexible settings, and immediate image viewing makes the BodyTom Elite a valuable tool to any facility needing versatile real-time mobile imaging.
About NeuroLogica
NeuroLogica Corp., the healthcare subsidiary of Samsung Electronics Co., Ltd., develops, manufactures, and markets innovative imaging technologies and is committed to delivering fast, easy and accurate diagnostic solutions to healthcare providers. NeuroLogica, the global corporate headquarters and manufacturer of mobile computed tomography, is also the US headquarters for sales, marketing and distribution of all Samsung digital radiography and ultrasound systems. NeuroLogica’s growing portfolio of advanced medical technologies is used worldwide in leading healthcare institutions helping providers enhance patient care, improve patient satisfaction, and increase workflow efficiency. For more information, please visit http://www.NeuroLogica.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211116005233/en/
CONTACT
Lynne Gagne
NeuroLogica
978.564.8576
lgagne@neurologica.com





