SEOUL, South Korea

CRScube Inc., one of the leading companies in Korea, is drawing attention in the global eClinical solutions market.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210914005075/en/
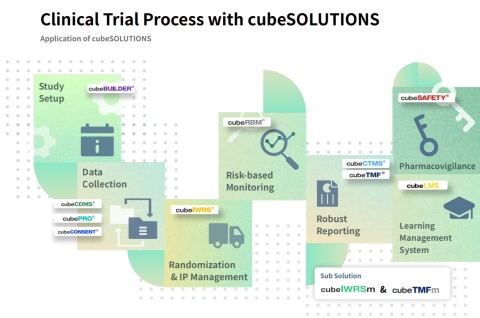
CRScube Inc. supplies essential software for all phases of clinical trials, ranging from design to data collection, reporting and managing patient results, and risk-based monitoring. CRScube offers a portfolio of solutions such as its flagship product, ‘cubeCDMS®,’ an Electronic Data Capture (EDC) solution for collecting clinical trial data; an integrated clinical trial management solution ‘cubeCTMS®’; a drug safety data management solution ‘cubeSAFETY®’; a patient data collection and result reporting solution ‘cubePRO®’; a clinical trial document management solution ‘cubeTMF®’; ‘cubeCONSENT®’ used for completing the patient consent process electronically; a learning/training management solution ‘cubeLMS®’. The company portfolio’s excellence in data sharing and interconnection of products enables users to execute and manage clinical trials efficiently. (Graphic: Business Wire)
The company is a healthcare software provider that develops cloud-based solutions and offers services used in clinical trials of medicines and medical devices. CRScube, founded in 2010, has supplied essential software for all phases of clinical trials, ranging from design to data collection, reporting and managing patient results, and risk-based monitoring. Particularly, excellence in data sharing and interconnection of products enables users to execute and manage clinical trials efficiently.
CRScube’s flagship product, ‘cubeCDMS®,’ an Electronic Data Capture (EDC) solution for collecting clinical trial data, has been used in over 3,000 clinical trials in 20 countries through its headquarters in Korea and overseas entities in the U.S., Japan, and China.
Additionally, CRScube offers a portfolio of solutions such as an integrated clinical trial management solution ‘cubeCTMS®’; a drug safety data management solution ‘cubeSAFETY®’; a patient data collection and result reporting solution ‘cubePRO®’; a clinical trial document management solution ‘cubeTMF®’; ‘cubeCONSENT®’ used for completing the patient consent process electronically; a learning/training management solution ‘cubeLMS®’.
The company provides an integrated system that handles data collection, electronic consent forms, supply chain management of clinical drugs, tools for document management, patient results reporting, and tools for clinical trial management.
CRScube has insured compliance with the industry’s stringent regulations by securing numerous certificates including CDISC ODM, ISO 9001, ISO 27001, FDA 21 CER Part 11, ISPE GAMP5.
CRScube’s CEO Kidon Kim said, “Our solution is an integrated system, which is developed on the basis of experience and feedback accumulated in the field, and global standards, and helps clinical trials of drugs and medical devices to be conducted in an efficient way,” adding “We are on the way to achieving our vision of developing clinical trial software and services that benefit everyone equally.”
CRScube participated in the development of healthcare services for the ‘Core Industry Cloud Flagship Project,’ that the National IT Industry Promotion Agency (NIPA) is dedicated to promoting the Korean ICT industry. NIPA proactively supports cloud-based service developers to accelerate the transition and expansion of the core industry into cloud-based services.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210914005075/en/
CONTACT
CRScube, Inc.
Kidon (Stanley) Kim
+82-2-722-7275
stanley@crscube.io





