MADISON, Wis.

Propeller Health today announced it will begin distributing in Japan, via a collaboration with Novartis to connect the Propeller digital health platform to Enerzair® and Atectura® Breezhaler®, two new once-daily Novartis medications available outside the U.S. to treat uncontrolled asthma.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200826005261/en/
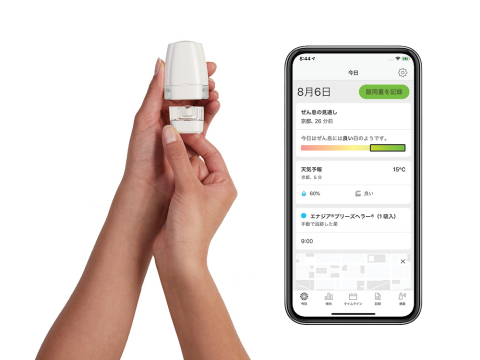
Propeller Health app and sensor with Breezhaler inhaler (Photo: Business Wire)
Patients using Enerzair® and Atectura® Breezhaler® to manage their uncontrolled asthma in Japan will have the option to enroll in Propeller’s digital health platform to help manage their condition.
Propeller’s platform works by attaching a sensor to the Enerzair® Breezhaler® or Atectura® Breezhaler® inhaler, which then delivers objective data on medication use to the Propeller app on the patient’s smartphone. The app also sends the patient reminders to take their prescribed dose and keeps a record of adherence data over time. The patient can share that data with their clinician to help inform the patient’s treatment plan.
“We are thrilled to bring the Propeller platform to Japan and introduce digital health for asthma to a large population of patients who can benefit from better management,” said David Van Sickle, co-founder and CEO of Propeller Health. “This collaboration is an important step in improving outcomes for people with chronic respiratory disease worldwide.”
Enerzair® Breezhaler® was approved in Japan on June 29 for the treatment of bronchial asthma (when combination of inhaled corticosteroids, inhaled long-acting β2-agonists, and inhaled long-acting anticholinergic agents is required). Atectura® Breezhaler® was also approved as a LABA/ICS combination asthma treatment.
In previous clinical studies unrelated to this partnership, the Propeller platform has been shown to increase asthma control by up to 63 percent,1 increase medication adherence by up to 58 percent,2 and reduce asthma-related emergency department visits and hospitalizations by as much as 57 percent.3
Propeller and Novartis first announced a collaboration to pair the Enerzair® Breezhaler® and Propeller digital health platform in Europe in July 2020.
1 Merchant RK et al. J Allergy Clin Immunol Pract 2016
2 Van Sickle D et al. Eur Respir J 2016
3 Merchant RK et al. World Allergy Org J 2018
About Propeller Health
Propeller Health is a leading digital health company dedicated to making life better for every person with chronic respiratory disease. Propeller creates products to more effectively treat chronic respiratory disease and improve clinical outcomes for patients through connectivity, analytics, and companion digital experiences. The Propeller platform is used by patients, physicians and healthcare organizations in the United States, Europe and Asia. Propeller Health is a wholly owned subsidiary of ResMed (NYSE: RMD, ASX: RMD). For more information, visit www.propellerhealth.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200826005261/en/
CONTACT
Rachel Fields
+1 630.901.8265
rachel.fields@propellerhealth.com





