TOKYO

Asahi Kasei has established industrial manufacturing technology for hyaluronic acid (HA) nanogel* as a new pharmaceutical excipient, and is providing samples to prospective customers in order to commercialize the product.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200804005334/en/
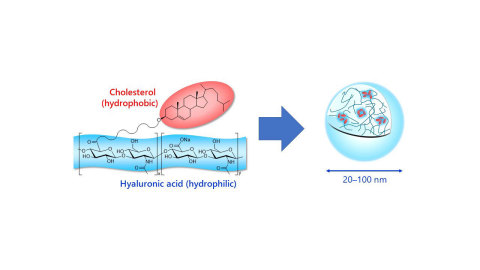
Chemical structure and self-assembly of HA nanogel (Graphic: Business Wire)
The Functional Additives Division of Asahi Kasei’s Specialty Solutions SBU supplies Ceolus™ microcrystalline cellulose to pharmaceutical manufacturers around the world for use as an excipient for drug tablets. The commercialization of HA nanogel would expand the product lineup by adding a drug delivery system (DDS) base material for injection formulations.
HA nanogel is expected to improve the quality of life (QOL) for patients by enabling the low-toxicity solubilization of insoluble drugs, facilitating the formulation of biopharmaceuticals such as proteins and peptides by suppressing their aggregation and denaturation, and reducing the frequency of injection when repeated administration is required.
Characteristics of the new HA nanogel:
Structure
HA nanogel is derived by the partial modification of hyaluronic acid with cholesterol. In water, self-association occurs among the cholesterol portions due to hydrophobic interaction, causing the formation of nanometer-scale hydrogel. The physical properties of HA nanogel depend on the molecular weight of HA and the cholesterol modification rate. Two grades of samples, a dispersion grade and a precipitation grade, are currently available.
Functions
The hydrophobic interaction enables a wide variety of insoluble drugs, ranging from low and medium molecular weight (MW) compounds to proteins, to be enclosed in the nanogel by simple mixing. This makes HA nanogel suitable for use as a base material for DDS. The main functions are sustained release, solubilization, suppression of aggregation, and retention of drug activity. Grades can be optimized to match specific customer requirements.
Asahi Kasei will make a formal decision on the commercialization of HA nanogel after confirming that the provided samples have contributed to solutions for the development of drug formulations by customers.
Asahi Kasei remains committed to meeting the needs of customers through products that contribute to life and living for people around the world.
Inquiries regarding HA nanogel samples should be directed to:
New Product Development Office
Functional Additives Division
Specialty Solutions SBU
Asahi Kasei Corp.
hananogel@om.asahi-kasei.co.jp
* Asahi Kasei has an exclusive license for HA nanogel technology developed by Chugai Pharmaceutical Co., Ltd.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200804005334/en/
CONTACT
For more information, please contact:
Specialty Solutions SBU
Functional Additives Division
Yurika Tanaka
tanaka.yz@om.asahi-kasei.co.jp
Phone: +81-(0)3-6699-6863
Asahi Kasei Corp.
Corporate Communications
Phone: +81-(0)3-6699-3008





